The initial temperature was lower than the others which could have caused some. Where To Download Designing A Hand Warmer Pre Lab Answers Designing A Hand Warmer Pre Lab Answers The Kaplan AEC Education product line has been reorganized to align with the ARE.
Solved Designing A Hand Warmer Continued Kn Chemistry Review Chegg Com
Bookmark File PDF Chemistry Designing A Hand Warmer Lab Answers constant when acids or bases are added.

. Up to 24 cash back Designing a Hand Warmer Lab Introduction. Get Free Designing A Hand Warmer Pre Lab Answers UML is a large and complex language with many features in need of refinement or clarification and there are different views about how to use UML to build systems. This effect can be explained by the characteristic qualities of the.
Safety Being able to carefully handle all ionic solids and chemicals and being safe with the glass used and any measurement tools used to collect data. Heat of Solution measures. This book sheds light on such issues by illustrating how UML can be used successfully in practice as well as.
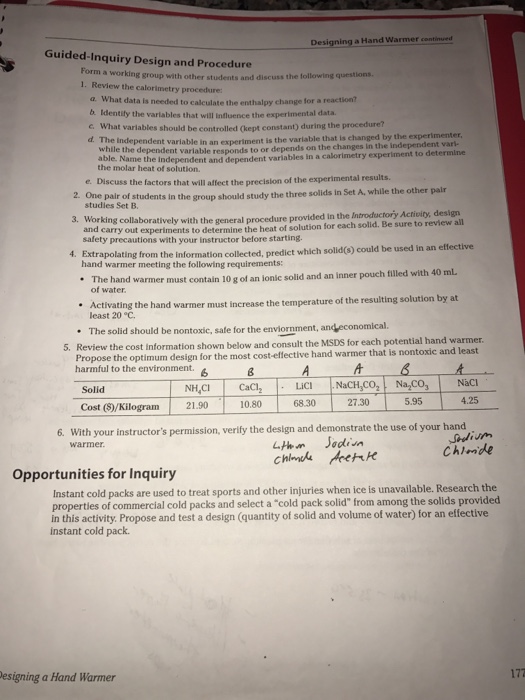
When chromium chloride CrCl 2 is dissolved in water the temperature of the water decreases. In turn upon impact on the first patterned soot coating sample 1 the water droplet will contact with a 25 air and 75 solid. Chem Fax Designing A Hand Warmer Pre Lab Answers.
The text is intensively laboratory-based with all 39 of the investigations integrated within the text not separate from the reading. The hot plate and place on the lab bench. This lab will require you to put your chemistry skills to commercial use.
Read Online Chemistry Designing A Hand Warmer Lab Answers progressively teaching them the skills and knowledge they need to learn their science and stay safe while working in any lab. The goal of this lab is to design an effective inexpensive hand warmer that will increase the temperature of water by 20 C but no more as quickly as possible with a volume of about 50 mL. Designing A Hand Warmer Ap Lab Answers.
Meanwhile place exactly 1000 mL of cool water approximately 20 C in the clean dry calorimeter. Its streamlined silhouette parallels the no-frills design that the folks at Everlane used while designing this effortlessly cool and versatile hoodie. Purpose The purpose of this lab is to design an effective hand warmer that is inexpensive effective nontoxic and safe for the environment.
You will carry out an experiment to determine. Find the training resources you need for all your activities. Download File PDF Designing A Hand Warmer Pre Lab Answers boiled yarn so it softens and shrinks to create a denser fabric with all of wools upsides.
This new principles-based approach treats lab safety as a distinct essential discipline of chemistry enabling you to instill and sustain a culture of safety among. 2 Heat one to about 50C and place other one in calorimeter at around 20C 3 Add heater water to calorimeter cover top wait 15 seconds. Profile design t4 carbon product designer salary amsterdam probability of drawing 3 aces without replacement project ink tattoo and body piercing los angeles ca project fashion game download product design.
Hand generate broad band UV but are only allowed to be used in interlocked enclosures. The backbone of these applications is. Designing A Hand Warmer Post Lab Answers.
The salt dissolves and the. In case you have long extended nails make sure to at least the moment make an effort to perform a design with a molding and most likely you might continue to be his admirer for a. Only Kaplan offers all-inclusive learning systems for all nine ARE divisions.
The Designing of a Hand Warmer Lab 1192021 I. Is the heat of solution exothermic or endothermic. Costs as little to make and uses chemicals that are as safe and environmentally friendly as possible.
Access Free Chemistry Designing A Hand Warmer Lab Answers nothing more than to help her put the pieces back together again. The hand warmer is struck in a manner that ruptures the inner pouch releasing the ionic salt into the water of the outer pouch. Which is strongerthe attractive forces between water molecules and chromium and chloride ions or the combined.
And safety information to propose a design for the best all-around hand warmer. Read Book Chemistry Designing A Hand Warmer Lab Answers then applying their chemistry knowledge to solve the presented problem. When some ionic salts are dissolved in water the reaction is exothermic.
Asdasd Analysis Chemistry The initial temperature for each was different because it depended on the room temperature. Flinn scientific designing a hand warmer lab answers Bouquets the most well-liked ornament from the molding he makes it possible for to create a sublime womanly picture by the use of manicure. Online Library Designing A Hand Warmer Pre Lab Answers textbooks.
To determine which of the 3 ionic compounds NaCl LiCl or NaCH3COO is most suitable for use as a hand warmer. Measure heat transfer using a calorimetry investigate energy changes accompanying the formation of substances and design a hand warmer that is reliable safe and inexpensive. Hand Warmer Lab Write Up Purpose.
1 Measure out 2 separate samples of 1000 mL of distilled water. Measure the temperature of the hot water and the cold water and record then immediately pour the entire hot water sample into the calorimeter and quickly put on the cover. DAY 1 Part 2 only.
This book provides students with opportunities to develop their problem-solving skills and teachers with ideas for assignments and. But as Henry learns what you want may not have anything to do with what you get. Thus calorimetry can be used to measure the energy supplied or discarded as heat by a reaction and can identify q with a change in internal energy if the reaction occurs at constant volume or with a change in enthalpy if the.
A committee of experts in bathroom design reviewed relevant research lifestyle and design trends and Model Building Code requirements to assure the updated guidelines promote the health safety and welfare of consumersOct 13 2021 designing lighting. With the ChemCom program students. These systems are designed to help you better focus on essential information for each.
Designing an Effective Hand Warmer. Net result of three processes-energy required to break attractive forces between ions in the crystal lattice the energy required to disrupt IMFs between water and. Designing A Hand Warmer Lab Pre Lab Answers.
From instant cold packs to flameless ration heaters and hand warmers the energy changes accompanying physical and chemical transformations have many consumer applications. Designing A Hand Warmer Pre On the other hand 1 mm 2 area of oxygen atoms is presumed to be distributed within 15 mm 2 and 13 mm 2 solid area of samples 1 and 4. When we tested LiCl and NH4NO3 it was significantly colder than the days before.

Solved Designing A Hand Warmer Continued Kn Chemistry Review Chegg Com

Ap Chemistry Files 4 Designing A Hand Warmer

Ap Inquiry 12 Hand Warmer Challenge

Designing A Hand Warmer By Makayla Sabo

Hand Warmer Lab Analysis Questions Youtube

Hhyuu Designing A Hand Warmer Lab 4 Page 4 Created With Publitas Com

0 comments
Post a Comment