So we go ahead and draw in heres one of our orbitals for carbon so thats an SP three hybrid orbital. All levels except the first have p orbitals.
I Draw The Shapes Of D Orbitals Sarthaks Econnect Largest Online Education Community
Explain Paulis exclusion principle.
. Orbitals i Explain what the terms penetration and shielding mean. Draw the shapes of s p and d orbitals. For example the sigma molecular orbital that serves to bond two fluorine atoms together is generated by the overlap of p-orbitals part A below and two sp 3 hybrid orbitals of carbon may combine to give a similar sigma orbital.
The largest database 1 of organic compounds lists about 10 million substances which include compounds originating from living organisms and those synthesized by chemists. 1 Draw a Lewis structure using the molecular formula. The d-orbitals where electron density is oriented along the axes d x 2-y 2 and d z 2 are repelled much more by the ligands while the orbitals d xy d xz d yz having electron density oriented in.
Salt atoms because of. Explore molecule shapes by building molecules in 3D. Some elements do not obey the octet rule because they have more than eight valence electrons they have a _____ valence shell.
Water atoms are smooth and slippery. In Chapter 7 we noted that alkanessaturated hydrocarbonshave relatively few important chemical properties other than that they undergo combustion and react with halogensUnsaturated hydrocarbonshydrocarbons with double or. List quantum numbers and discuss their significance.
Our modern society is based to a large degree on the chemicals we discuss in this chapter. Draw probability pictures of an electron in an atom. Electron density diminishes gradually with increasing distance which makes it impossible to draw a sharp line marking the boundary of an atom.
This point is illustrated in Figure PageIndex1 which shows a plot of total electron density for all occupied orbitals for three noble gases as a function of their distance from the nucleus. So were gonna go ahead and draw in our carbon and we know that it has four SP three hybrid orbitals and once again when we draw the orbitals were gonna ignore the smaller back lobe here so it doesnt confuse us. Electrons in the same subshell have the same energy while electrons in different shells or subshells have different energies.
31 Fundamental Particles of Atom. The existence of so many organic molecules is a consequence of the ability of carbon atoms. In the case of bonds between second period elements p-orbitals or hybrid atomic orbitals having p-orbital character are used to form molecular orbitals.
Most are made from petroleum. Electron shells consist of one or more subshells and subshells consist of one or more atomic orbitals. Find out by adding single double or triple bonds and lone pairs to the central atom.
2 Count the number of electron groups surrounding the central atom 3 The number of bonding orbitals required is equal to the number of electron groups 4 Build the appropriate combination of atomic orbitals based on the number of bonding orbitals required. Define Aufbau principle and explain Hunds rule of maximum multiplicity. At the third level there is a set of five d orbitals with complicated shapes and names as well as the 3s and 3p orbitals 3px 3py 3pz.
Draw the d atomic orbitals as enclosed surfaces showing the sign of the original wavefunction on each lobe. Thus iron atoms are solid and strong with hooks that lock them into a solid. The Oxford Solid State Basics Solutions to Exercises.
In addition to s and p orbitals there are two other sets of orbitals which become available for electrons to inhabit at higher energy levels. The electrons in an atom are arranged in shells that surround the nucleus with each successive shell being farther from the nucleus. You have already seen that butane C 4 H 10 has an isomer 2-methylpentaneThese are the only two isomers of this molecule.
There is no number of chain isomers formula for alkanes and the number quickly grows cumbersome for example decane or C 10 H 22 has a whopping 75 isomers. The number of potential organic compounds has been estimated 2 at 10 60 an astronomically high number. This is only possible for elements that have available _____ orbitals elements from period _____ of the periodic table onward.
Ii How do these concepts help to explain the structure of the periodic table. Then compare the model to real molecules. Shapes of d-orbitals CRYSTAL FIELD EFFECTS ON OCTAHEDRAL COMPLEXES In octahedral complexes the ligands approach along the axes.
C 5 H 10 on the other hand has three isomers while C 6 H 14 has nine. The earliest views on the shapes and connectivity of atoms was that proposed by Leucippus Democritus and Epicurus who reasoned that the solidness of the material corresponded to the shape of the atoms involved. How does molecule shape change with different numbers of bonds and electron pairs.

Draw The Shapes Of Various P And D Orbitals Youtube

23 6 3 Shapes Of D Orbitals Youtube
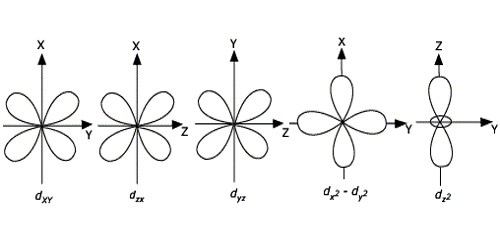
Explain Shape Of D Orbitals Qs Study
Draw The Shapes Of D Orbitals Heisenberg S Uncertainty Principle Sarthaks Econnect Largest Online Education Community

Draw The Shapes Of Five D Orbitals

Draw The Shapes Of Various P And D Orbitals Youtube
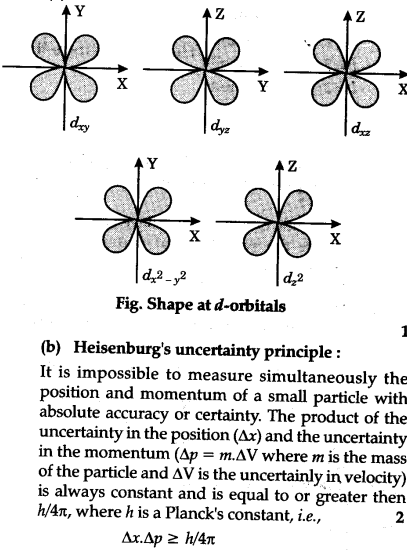
Draw The Shapes Of D Orbitals Cbse Class 11 Chemistry Learn Cbse Forum

0 comments
Post a Comment